PharmaShots Weekly Snapshots (June 17 – June 21, 2024)
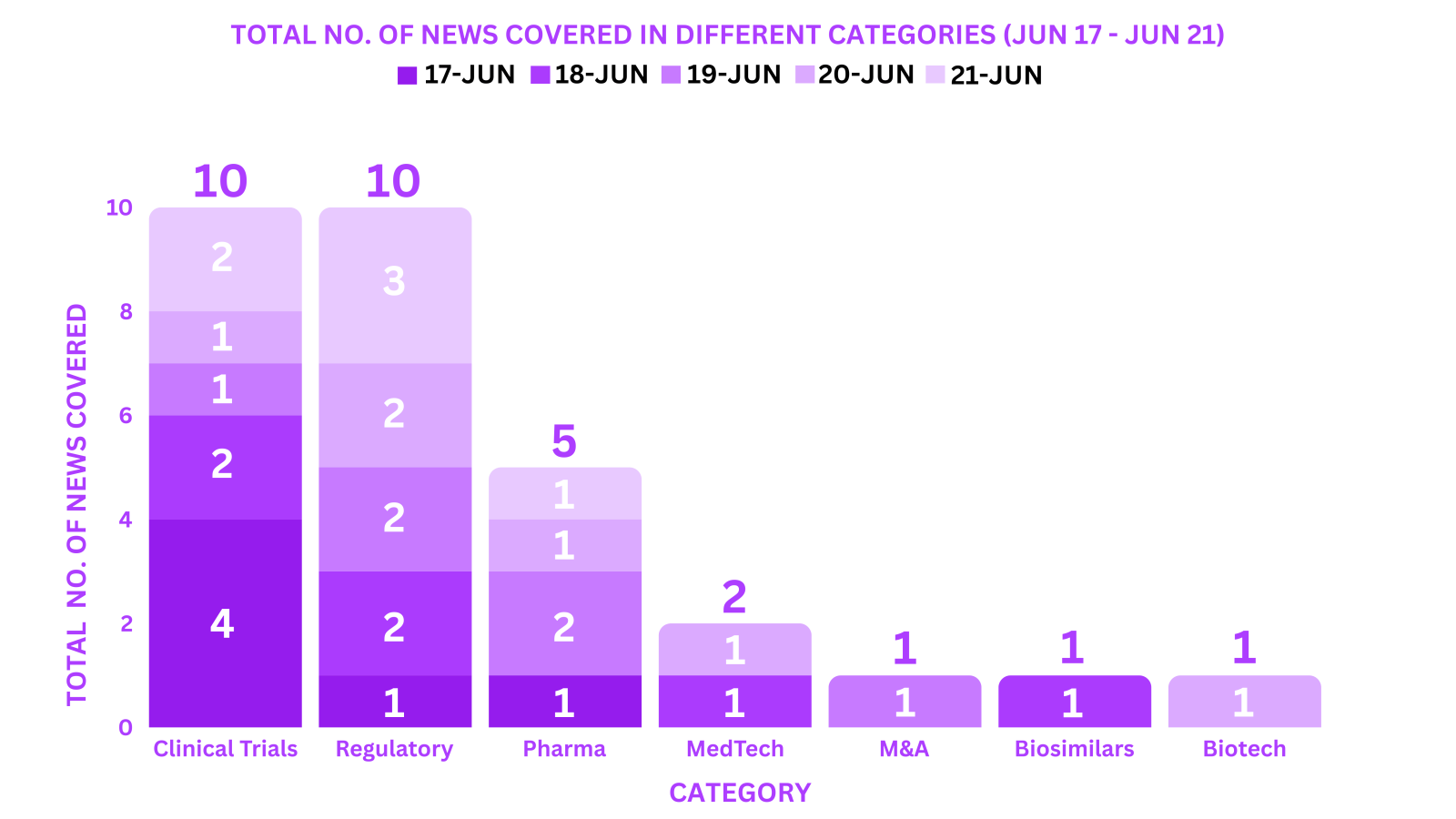
This week PharmaShots’ news was all about the updates on Clinical Trials, Pharma, Biotech, COVID-19, Regulatory & MedTech. Check out our full report below:
Roche Highlights Data from the P-III (STARGLO) Trial of Columvi to Treat Diffuse Large B-Cell Lymphoma at EHA 2024
Read More: Roche
AstraZeneca Showcases the P-III (ECHO) Study Data of Calquence Combination Regimen for Mantle Cell Lymphoma at EHA 2024
Read More: AstraZeneca
HUTCHMED Features the P-III (ESLIM-01) Study Data of Sovleplenib for Primary Immune Thrombocytopenia at EHA 2024
Read More: HUTCHMED
Regeneron Showcases Updated P-I/II (LINKER-MM1) Study Data of Linvoseltamab for Treating R/R Multiple Myeloma at EHA 2024
Read More: Regeneron
AstraZeneca’s Imfinzi Combined with Chemotherapy Gains the US FDA’s Approval to Treat Endometrial Cancer
Read More: AstraZeneca
Takeda Reports Topline Data from P-III (SKYLINE and SKYWAY) Studies of Soticlestat for Dravet Syndrome (DS) and Lennox-Gastaut Syndrome (LGS)
Read More: Takeda
AstraZeneca Reports Data from the P-III (CAPItello-290) Study of Truqap Combined with Chemotherapy to Treat Triple-Negative Breast Cancer
Read More: Astrazeneca
Tubulis Reports the First Patient Dosing with TUB-040 in P-I/IIa Study to Treat Ovarian Cancer and Lung Adenocarcinoma
Read More: Tubulis
Neumora Therapeutics Commences the P-Ib Trial of NMRA-511 to Treat Agitation Due to Alzheimer’s Disease
Read More: Neumora Therapeutics
Aphaia Pharma Reports the P-II Study Results of APHD-012 Among Pre-Diabetic Individuals
Read More: Aphaia Pharma

Amgen’s Blincyto (Blinatumomab) Receives the US FDA’s Approval to Treat B-Cell Precursor Acute Lymphoblastic Leukemia (B-All)
Read More: Amgen
Merck’s Capvaxive Receives the US FDA’s Accelerated Approval to Prevent Invasive Pneumococcal Disease and Pneumonia in Adults
Read More: Merck
Johnson & Johnson Reports the BLA Submission of SC Amivantamab to the US FDA for Treating Non-Small Cell Lung Cancer
Read More: Johnson & Johnson
AbbVie's Skyrizi Gains the US FDA’s Approval for Treating Ulcerative Colitis
Read More: AbbVie
Sobi’s Altuvoct Receives the EC’s Marketing Authorization to Treat Haemophilia A
Read More: Sobi
AstraZeneca’s Truqap in Combination with Faslodex Receives EC’s Approval for Treating Breast Cancer
Read More: AstraZeneca
Dizal’s Golidocitinib Gains the NMPA’s Approval to Treat Peripheral T-Cell Lymphoma
Read More: Dizal
Sarepta Therapeutics’ Elevidys Gains the US FDA’s Label Expansion Approval for Duchenne Muscular Dystrophy
Read More: Sarepta Therapeutics
Johnson & Johnson Reports sBLA Submission of Tremfya to the US FDA for Treating Crohn’s Disease
Read More: Johnson & Johnson
BMS Reports the EMA’s Validation of the Application for Subcutaneous Opdivo
Read More: BMS

Takeda Enters into an Option Agreement with Ascentage Pharma for Olverembatinib to Treat Chronic Myeloid Leukemia
Read More: Takeda & Ascentage Pharma
Ascidian Therapeutics Join Hands with Roche to Discover and Develop RNA Exon Editing Therapies for Neurological Diseases
Read More: Ascidian Therapeutics & Roche
Belharra Therapeutics Partners with Sanofi to Discover New Small Molecules Addressing Immunological Diseases
Read More: Belharra Therapeutics & Sanofi
Biophytis and Blanver Collaborate to Develop BIO101 for Treating Multiple Indications
Read More: Biophytis & Blanver
Alivexis and Melodia Therapeutics Join Forces to Develop MDI-0151 for Inflammatory Diseases
Read More: Alivexis & Melodia Therapeutics

Alvotech and Advanz Pharma Team Up to Commercialize AVT06/AVT29 (Biosimilar, Eylea) in Europe
Read More: Alvotech & Advanz

Roche’s Digital Pathology Solution Bags the US FDA’s Clearance for its Use in Diagnosis
Read More: Roche
Roche Introduces In-Situ Hybridisation (ISH) Test for the Diagnosis of B-cell Lymphoma
Read More: Roche

Boston Scientific Reports the Acquisition of Silk Road Medical
Read More: Boston Scientific & Silk Road Medical

OSE Immunotherapeutics Highlights Preclinical Data of mRNA Therapeutic Platform to Treat Inflammatory and Autoimmune Disorders at FOCIS 2024
Read More: OSE Immunotherapeutics
Related Post: PharmaShots Weekly Snapshots (June 10 – June 14, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.